

TOPIC: Introduction, Allotropes and Properties
Carbon, a chemical element, has the symbol C with 6 as
the atomic number. Carbon has a unique property to form bonds with
other carbon atoms to form long chains, which may be straight,
branched or even rings.
The carbon atoms may be linked by double
or triple bond in addition to single bonds.
The carbon-carbon bond
is very strong and hence stable. Carbon is also tetravalent. Because
of these reasons, the number of carbon compounds is so large.
Let us study some more terms related to Carbon and the compounds
formed by it.
Allotropes: The existence of
elements in two or more different forms having different physical
properties but with identical chemical properties.
Allotropism: The phenomenon of existence of an element
in two or more physically different forms but with similar chemical
properties.
Diamond: An allotrope of Carbon, in
which each carbon atom lies at the centre of a regular tetrahedron and
is covalently bonded with four carbon atoms located at the four
corners of the regular tetrahedron.
Diamond
 Figure: Diamond
Figure: Diamond
 Figure: Graphite
Figure: Graphite
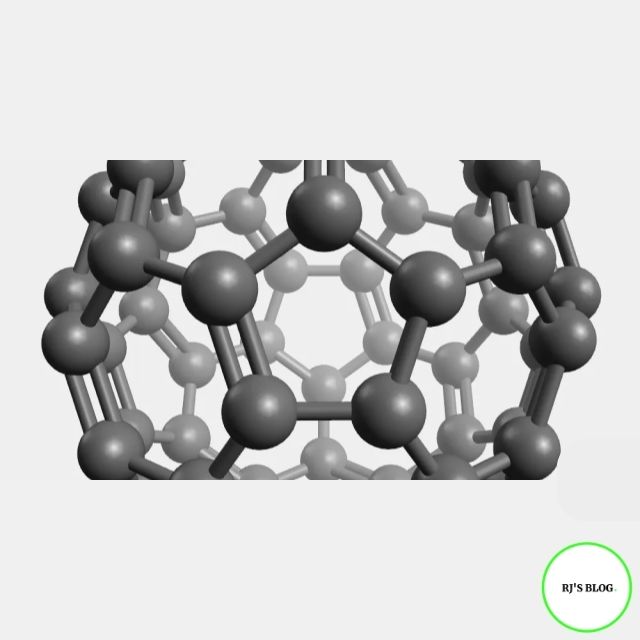 Figure: Fullerene
Figure: Fullerene
Team Unity and Team Chemistry is by far more important than talent
- Rob Colbert